By Steve Voynick
New Mexico’s Harding Mine, aka the Harding Pegmatite, is a popular destination for mineral collectors, amateur geologists, mining historians, and geology students on university field trips. Several thousand visit the mine each year to collect unusual minerals, study a textbook example of a zoned granite pegmatite, and learn about a little-known side of mining history.
Owned and managed by the University of New Mexico (UNM), the Harding Mine is hidden away in the piñon-juniper woodlands of northern New Mexico’s Picuris Mountains east of the town of Dixon. The mine is 54 road miles north of Santa Fe and 33 miles south of Taos. Despite being a bit off the beaten path, it’s well worth a visit by anyone interested in minerals, geology, and mining history.
Pegmatite’s Mineralogical Importance

The term “pegmatite” refers to any extremely coarse-grained igneous rock, or to a body of such rock in which individual crystals exceed one centimeter (0.4 inches) in diameter. Granite pegmatites are the most common and have the greatest economic and mineralogical importance. Granite consists mainly of quartz and various feldspar-group and mica-group minerals. Intruded granitic magma usually solidifies relatively quickly to form common granite with its uniform, evenly dispersed, individual crystals, all less than one centimeter in size.
But when isolated pockets of magma, called residual magma, retain heat and cool slowly, they solidify in a process of fractional crystallization. Rather than quickly “freezing” into common granite, residual magma solidifies very slowly on a mineral-by-mineral basis into the large, well-formed crystals typical of pegmatites. As the last of the original magmatic mass to solidify, residual magma often contains concentrations of rare and unusual elements and minerals. Granite pegmatites can form within a greater mass of common granite or, in the case of the Harding Pegmatite, within a different type of host rock. The latter occurs when granitic intrusions cool and contract to literally “squeeze” residual magma into fissures of adjoining, non-granitic country rock.
Granite pegmatites occur as horizontal lenses, pods, disks, erratic dikes, or veins, or combinations of thereof. Pegmatite bodies range in length from only a few feet to more than a mile. Because pockets of residual magma cool slowly from the outside inward, fractional crystallization sometimes creates a well-defined, concentric structure of different mineral zones. The Harding Pegmatite has eight distinct zones, each classified by their primary mineral or minerals. Lithium is present throughout the pegmatite in concentrations well above the crustal average.
Complexly structured and irregularly shaped, the Harding Pegmatite is 2,500 feet long with a maximum width of 500 feet. Radiometric dating indicates it was emplaced at a depth of about seven miles during the mid-Precambrian Era some 1.3 billion years ago. Regional uplifting and subsequent erosion eventually exposed roughly of the pegmatite’s length.
Diverse Mineral Representation
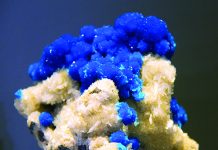
Mineralogists have identified more than 60 minerals at the Harding Pegmatite. In order of abundance, the major minerals are quartz (silicon dioxide, SiO2) and the feldspar-group minerals albite (sodium aluminum silicate, NaAlSi3O8) and microcline (potassium aluminum silicate, KAlSi3O8). The quartz occurs mostly in milky-white masses, the feldspars as fine-grained, white aggregates. Next in abundance is muscovite or basic potassium aluminum silicate, KAl3Si3O10(OH)2, the most common mica-group mineral. Some occur as pale, yellow-to-greenish flakes up to an inch in size; most, however, appears as a pink, lithium-rich variety called “rose muscovite.” Rose muscovite contains 3.5-4.6 percent lithium oxide (Li2O) and forms aggregates of tiny, pink crystals.
Also abundant is lepidolite, or potassium lithium aluminum fluorosilicate, a mica-group

mineral found only in lithium-rich granite pegmatites. With a Mohs hardness of 2.5-3.0 and a specific gravity of 2.8, lepidolite crystallizes in the monoclinic system as micaceous masses of small flakes and fine-grained aggregates of tiny, glittery crystals with a pearly luster and purplish colors. Another important mineral is spodumene or lithium aluminum silicate, LiAl(Si2O6), which contains 6.8 percent lithium oxide and crystallizes in the monoclinic system. At the Harding Pegmatite, spodumene occurs as both fine-grained masses and tapering, lath-shaped, opaque, white-to-light-gray crystals several feet in length.
Among the accessory minerals are beryl and microlite. Beryl or beryllium aluminum silicate, Be3Al2(Si6O18), contains five percent beryllium and crystallizes in the hexagonal system. It has a Mohs hardness of 7.5, a specific gravity of 2.75, and a resinous luster. The beryl at the Harding Pegmatite forms fine-grained masses and coarse, opaque, off-white crystals of varying size. Microlite, a rare sodium-calcium tantalite containing 68 percent tantalum and small amounts of niobium and uranium, crystallizes in the cubic system as sub-translucent-to-opaque octahedral crystals, aggregates of small crystals, and tiny, disseminated crystals. It has a specific gravity of 5.0, Mohs hardness of 5.5, and colors ranging from pale yellow to reddish-brown and black. Its small uranium content makes microlite measurably radioactive.
(Note: The International Mineralogical Association has discredited lepidolite and microlite as species. Lepidolite is now part of the polylithionite-trilithionite series, while microlite is a member of the pyrochlore-microlite series. But because their names remain in common usage and appear in most Harding Pegmatite literature, I am using them in this article.)
The economic minerals at the Harding Pegmatite were lepidolite, rose muscovite, and spodumene, valuable for their lithium content; microlite, for tantalum; and beryl, for beryllium. Reflective outcrops of light-colored minerals had been attracting prospectors to the Harding Pegmatite since the 1870s. But it wasn’t until 1918 that exposed masses of lilac-colored rock were identified as lepidolite. Lithium, then in short supply, was needed for special types of glass, including the newly introduced, heat-resistant PYREX™ line of kitchenware and bakeware.
Harding Mine’s Many Contributions to Industrial History
Soon after mining began in 1919, the Harding Mine was shipping 800 tons of hand-cobbed lepidolite annually, along with smaller amounts of rose muscovite and spodumene. Then in 1924, two shipments were found to contain a mineral contaminant that was detrimental to glass-making. Mine production was suspended for a year until the contaminant was identified as microlite, which was then manually removed from further lepidolite shipments. The Harding Mine was only marginally profitable and closed in 1930 when the richest lepidolite ore was depleted. By then it had shipped 13,500 tons of lithium-bearing minerals, mostly lepidolite, worth $140,000.

In 1931, a prospector found a shallow occurrence of optical-quality “Iceland spar”—the doubly refractive, transparent variety of calcite—just west of the Harding Pegmatite but not geologically related to it. For several years, a small, two-man, open-pit operation recovered Iceland spar crystals, some weighing up to five pounds, and shipped them to optics-manufacturer Bausch & Lomb to cut into prisms for use in optical range-finding instruments.
The Harding Mine remained inactive through the 1930s but again drew attention at the start of World War II when the United States government was desperately searching for tantalum sources. A rare, dense, blue-gray, corrosion-resistant metal with unusual electrical properties, tantalum was used with capacitors for the battlefield and aircraft radios, as a component of alloys for experimental jet engines, and as a surgical cranioplasty-implant material for the treatment of combat-related head wounds. Due to wartime demand, the price of tantalum mine concentrate soared sixfold to $3.50 per pound.
Arthur Montgomery, a 33-year-old geologist and mineral collector, investigated the Harding Pegmatite in 1942 and found concentrations of previously unwanted microlite. After a United States Geological Survey core-drilling team confirmed his findings, Montgomery signed a lease-purchase agreement for the property and began mining. Because of microlite’s high value and disseminated nature, blasting was not an option. The six miners that Montgomery hired from nearby Dixon mined only with hammers and chisels, then hand-concentrated the ore. Identifying the tiny, pale-yellow crystals was difficult visually, but Montgomery taught his crew to recognize two of microlite’s physical properties—its unusual density and subsequent “heft,” and its very high index of refraction that revealed its crystal outlines when moistened with saliva.
Montgomery also mined spodumene for its lithium content, then needed for submarine air-purification systems and inflatable flotation devices. He also mined beryl for its beryllium. Neither Montgomery nor his miners knew what the beryllium was being used for. Only after the war had ended did they learn that it had been fabricated into vital neutron reflectors for the top-secret development of the atomic bomb at nearby Los Alamos.
Supporting Wartime Efforts
But the mine’s most important wartime mineral product was still microlite concentrate—more than 6,000 pounds in 1943 alone. The Harding Mine was the nation’s biggest source of tantalum, and by the time mining ceased in 1947, it had supplied 22,000 pounds of microlite concentrate, along with 1,000 tons of beryllium- and lithium-bearing concentrates. Montgomery’s substantial profit enabled him to purchase the mine, which he reopened in 1949, this time exclusively for beryl to ship to what had then become the Los Alamos Scientific Laboratory. Montgomery’s mining team consisted of himself, five Dixon miners, and a “twelve-dollar” mule fittingly named Beryl. Because beryl (the mineral, not the mule) was easily confused with microcline, albite, and spodumene, Montgomery taught his miners to recognize it by its resinous luster.
By the time the mine closed permanently in 1958, it had produced 900 tons of hand-concentrated beryl for Los Alamos. The mine again made a nice profit, part of which Montgomery contributed to building a public school in Dixon.
After earning a Ph.D. in mineralogy from Harvard University, Montgomery became a

college professor and remained active in the world of minerals. In 1970, he helped found the Friends of Mineralogy and provided part of the funding to establish The Mineralogical Record. Montgomery also hoped to preserve the Harding Pegmatite for its educational and research value. Upon retiring from teaching in 1974, he offered the mine to UNM. But the property included both patented and unpatented claims, which complicated the transfer of land to the state. The transfer’s legality was also questioned because of the strategic importance of the mine’s remaining reserves of tantalum and beryllium.
Arranging the transfer took four years and required congressional approval. The government retained the right in perpetuity to mine the strategic resources if necessary in the future. Finally, open to the public, the Harding Pegmatite hosted 500 visitors in its first year. As this number steadily increased, the mine’s underground workings, popular with visitors but increasingly unstable, became a safety concern. In 2011, the New Mexico Department of Energy, Minerals and Natural Resources committed $207,000 to the Harding Pegmatite Mine Safeguard Project. Workers installed specially designed steel grates to seal off the underground areas but leaving them accessible to qualified researchers and the region’s large summertime bat population. They also installed interpretive signs and steel marker posts along a self-guided walking-tour route.
One of the first things that visitors notice upon entering the Harding Pegmatite is the exposed, sheer west wall, where a mass of white pegmatite minerals contrasts sharply with the dark host rock of metamorphic amphibolite. This well-defined upper limit of the pegmatite helps to visualize its original, pre-mining configuration. The abundance of lepidolite and rose muscovite imparts delicate pink-purple colors to most exposed rock surfaces. Although it is difficult to differentiate these minerals because of their color, grade, and overlap, the general rule at the Harding Pegmatite is to attribute the purples to lepidolite and the more pinkish hues to rose muscovite.
Because lithium is not a chromophoric element, it does not impart these pink-purple colors. These colors are actually due to traces of manganese, which is present not only in lepidolite and rose muscovite, but also as prominent dendritic patterns of black manganese oxides on cleavage surfaces of light-colored feldspar minerals. Interpretive signs highlight the Harding Pegmatite’s mining history, geology, and the safeguarding project that sealed the underground workings. In the body of massive, white quartz above one of the steel grates are the impressions of three-foot-long, lath-like spodumene crystals that were mined for their lithium content.
Drawn In By A Pinnacle

Of particular interest along the walking-tour route is a 15-foot-high pinnacle that miners left in place. The top is solid beryl and the middle massive quartz, while the base is “spotted rock,” consisting of quartz and microcline, both a delicate pink-purple. The base rock is distinctive for its “polka-dot” pattern of two to three-inch white “spots” of spodumene. Throughout the spotted rock are tiny crystals of brownish-black microlite, the wartime tantalum ore. Radiation emitted by the microlite’s uranium component is easily detectable with a field radiation monitor. These microlite crystals were originally embedded in white quartz. Radiation has since altered this to smoky quartz, which appears as a small, but distinct, amber-colored “halo” around each microlite crystal.
Collecting at the Harding Pegmatite is more a matter of selecting specimens than searching for them. Experienced collectors often focus on the extensive mines dumps, which require no work with rock picks, hammers, and chisels. Collecting at this site requires some common-sense caution. Potential hazards include steep and unstable terrain, rockfall from the sheer walls, and the occasional rattlesnake. Cell phone service can be spotty, and Dixon is some distance away. Good sunglasses will also make a day at the Harding Pegmatite more enjoyable. The exposed rock within the pegmatite consists entirely of highly reflective, white, or light-colored minerals. Surface glare on sunny days, which means most days in northern New Mexico, can quickly lead to pounding headaches for unprepared collectors.
UNM’s Department of Earth and Planetary Sciences manages the Harding Pegmatite and has established a visitation protocol. All visitors are required to sign two copies of a liability disclaimer; one must be mailed to the UNM department office in Albuquerque, the other carried on your person while at the mine. By signing this form, visitors acknowledge the risks involved and release UNM from any liability.
This website also provides a downloadable walking-tour map of the Harding Pegmatite, highway directions, and a detailed geological and mineralogical guide to the numbered stops along the walking tour route. UNM limits individual collectors to five pounds of specimens—a reasonable request considering that the mine now hosts about 3,500 visitors per year.
The Harding Pegmatite is located six miles east of Dixon, just off New Mexico Route 75. The mine cannot be seen from the highway and the unmarked turnoff onto a quarter-mile-long dirt access road is easy to miss. Locked gates may necessitate a short walk to the mine. Follow the highway directions posted on the website. The mine’s caretaker lives near Dixon and can accompany group tours upon prior arrangement.
Although it’s out of the way, the Harding Pegmatite is well worth a visit. You’ll come away with an understanding of zoned granite pegmatites and an unusual bit of mining history—along with a nice collection of colorful and interesting pegmatite minerals.