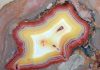
Calcite is an ideal mineral for collectors who are just starting and for seasoned hobbyists who want to refine their focus. Beginner collectors quickly realize there are two challenges in mineral collecting: too many minerals and too few dollars. For most of us, those challenges never disappear; we simply learn how to manage them.
One effective solution is to specialize. I found I couldn’t collect worldwide species and still maintain the quality I wanted, so I narrowed my focus to two localities—Mexico and Arizona. Mexico was my choice because it produces abundant, high-quality minerals and lies just across the border from my home in Arizona. I also emphasized two mineral species common to both areas, copper and lead, and focused on miniature specimens, which are often more affordable.
Calcite as a Collector’s Starting Point
Calcite may be a great way to start collecting. By choosing calcite, it may seem simple, but it can be as complicated as you want to make it. You may discover a whole range of mineralogical and historical studies if you so choose.
You may not realize it, but calcite is the gateway to collecting many other species. It’s a great way to learn about crystal forms and the conditions where they develop. Studying calcite is the first step in understanding the world of mineralogy and a whole series of attractive and popular species.
Understanding the Calcite Mineral
Calcite is one of a group of related minerals. Calcite is calcium carbonate, but so is aragonite. Aragonite and calcite are polymorphs, meaning they have the same chemical elements, calcium and the carbonate radical.
The difference is in their crystal habit and form. Aragonite crystals are orthorhombic. Calcite is hexagonal. Normally, they look different from each other, but Mother Nature is not so simple. Both can develop as rhombic crystals, which look like a slanted box lacking right angles. You can not tell the difference.
To make things more complicated, Mother Nature can change aragonite crystals internally. Heat an aragonite rhombic crystal to about 300°F, and the internal structure changes to hexagonal calcite but keeps the original outer shape.
How Temperature and Pressure Shape Calcite Crystals
This brings up the effects temperature and pressure can have on crystal growth. At ambient pressures and temperatures that we normally enjoy, calcite forms in the familiar scalemohedral or dog tooth crystals. These are slender crystals that terminate to a sharp point. Dogtooth calcites are common in sedimentary rocks where temperatures and pressures are what we experience each day. As rains fall, particularly acid rain, it absorbs calcium carbonate, and when these solutions settle in sedimentary rocks, dogtooth calcite crystals may form.
As developing crystals form deep in the earth, they are subjected to high heat and pressure and crystals develop more complicated forms like rhombs or hexagons with rhombic terminations. Within limits, you can tell something about what the conditions were like where the crystals formed. Fluorite does the same thing. We find lovely cubes in the sedimentary rocks of the Midwest. In the deep hydrothermal sulfide deposits in China, Germany, and elsewhere, fluorite crystals develop a wide range of forms, with some crystals having as many as 48 faces.
Calcite from Superior, Arizona
On one of my underground visits, I was in the metal mine at Superior, Arizona. At a depth of 4800 feet, the rock wall was about 148°F, and pressures were up. The calcites found in this mine were wonderful complex rhombs, often twinned with curving modified faces. They were completely different from dogtooth calcite crystals found on the surface in Arizona.
Because of the dramatic differences in crystal forms, members of this group are in a subsystem, trigonal, which has been added to explain their common rhombic form. Another name assigned to this group is rhombohedral, since they all can form rhombic crystals.
Improving Your Mineral Collection Through Focus
By choosing one mineral to concentrate on, you can improve the quality of your collection, which is more important than quantity. You can also study an area more efficiently and in greater depth. You’ll find yourself becoming more interested in the history of certain localities, mines or countries.
Building a Collection by Locality
Collectors who choose to focus on a locality rather than a mineral tend toward the better-known sources. My son Evan has studied the history, geology and mineralogy of Bisbee because of his interest in copper minerals. If you hand him a specimen from Bisbee, the first thing he does is look at the matrix, not the crystals. It is the matrix that tells you the circumstances and geology of where and how the specimen developed. He can usually tell you where the specimen came from and maybe even the level in that mine where it formed.
Why Collectors Focus on Famous Localities
The advantage of collecting a locality is that it gives you a broader subject with a greater variety of minerals, mining techniques and environmental effects. You will get into the history related to the deposit, when it was found and by whom.
Among the localities, collectors usually pick deposits that have been exceptionally fruitful for specimens. The most popular are Bisbee, Cornwall, England, Michigan copper deposits, the Tristate area in the Midwest, the gem pegmatite deposits of Maine, California, Tsumeb, Namibia and the gem pegmatites in Brazil.
I had a particular interest in an old French copper mine in Chessy, France. Historically, Chessy produced superb copper species like cuprite, azurite and malachite. In its heyday, Chessy was also a major source of azurite that was crushed to make blue pigment for the artists in Europe.
Your interest in minerals will take you into all sorts of other subjects once you get started. Think of the many opportunities that will expand your interests and open your mind to the amazing world of mineral collecting. It’s a great hobby!
Calcite Collecting FAQ
Why is calcite a good mineral for beginner collectors?
Calcite is abundant, affordable, and forms a wide variety of crystal shapes. Collecting calcite helps beginners learn crystal habits, growth conditions, and basic mineralogy without a large investment.
What makes calcite crystals so different from one another?
Calcite crystal shapes are influenced by temperature and pressure during formation. Low-temperature environments often produce dogtooth crystals, while deeper, high-pressure settings create complex rhombs and modified forms.
Is calcite the same as aragonite?
No, but they are closely related. Both are polymorphs—minerals with the same chemical composition but different crystal structures and growth habits.
Should collectors focus on one mineral or one locality?
Both approaches work. Focusing on a single mineral builds depth of knowledge, while collecting by locality offers broader exposure to geology, mining history, and associated minerals.
This story about choosing what to minerals to collect previously appeared in Rock & Gem magazine. Click here to subscribe. Story by Bob Jones.