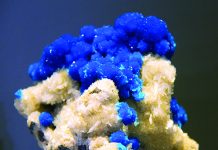
Aragonite and calcite are both members of the calcium carbonate group both with exactly the same chemical molecules in the same ratios but they are polymorphs each having different crystal structures. Calcite forms six-sided hexagonal crystals while aragonite forms four-sided orthorhombic beauties.
To confuse things further, aragonite has a great penchant for forming twins that look exactly like hexagonal crystals. It commonly forms in twins that join to form a six-sided crystal called a sixling, which may look like calcite. These aragonite sixling twins are pseudohexagonal. The key is pseudo, which means “false.”

Aragonite is less common than calcite, but in the organic world, it is very common and very important because it is an integral part of most seashells, pearls and ancient fossils, especially ancient ammonites.
Aragonite Formation
Aragonite and calcite form under low temperatures, but aragonite is a little less stable. After it forms, it does not take much for it to rearrange its structure and become calcite. For example, banded onyx, which is common around hot springs, often forms as aragonite but then tends to alter into calcite once cooled. This explains why we use the term calcite onyx.

Aragonite is a wonderful collector mineral because it is common enough to be found in quantity. It develops in a variety of attractive crystals. Some are long, tapered beauties that approach needle-like form. Such superb slender specimens were found in Podrecony, Slovakia. These crystals measured as much as six inches long and were snow-white, tapered, and randomly arranged in clusters.
These are among some of the finest specimens found in the last decades.
Tarnowice (Tarnowitz), Poland, has also been a fine source of long, slender aragonite crystals. Some of the largest aragonite crystals, up to a foot long, have come from Weitendorf, Styria, Austria. Fine examples of small, slender, needle-like crystals were once common from the iron mines at Frizington, Cumberland, England, where they occurred in crystals an inch or so in tight clusters covering matrix.
Flowers of Iron
The iron mines of Cumberland, England, also produced a really attractive form of aragonite that has been given several names including coralloidal. When first discovered in the mines of Leogang, Austria, it was called flos-ferri, or “flowers of iron.” These flowers of iron contain no iron. They are snow-white, twisting, curving, slender fingers of calcium carbonate aragonite.
Large specimens of this oddly formed aragonite show no crystal faces but are present in attractive twisted forms. Many very well-known mines have been a source of these unusual-looking but fascinating specimens. The copper mines of Bisbee, Arizona, and Santa Rita, New Mexico, have also been sources of flos-ferri, which is a pretty inexpensive addition to any collection.
Tarnowitzite
Earlier, the locality Tarnowice, Poland, was mentioned. Crystallized aragonite here was first thought to be a distinct new species containing lead, so it was named tarnowitzite, a now discredited name I still use.
Lead is not the only element that substitutes for calcium in aragonite. Nicholsonite is another variety of aragonite in which zinc is substituted for calcium.
Crystal Clusters
Yet another form of aragonite is found as small clusters of usually brown pseudohexagonal crystals locked together and pointing off in every direction. The crystals are seldom over an inch long, so crystal clusters are only a few inches across. Their brown color has to do with the earthy clay deposits where it is often found.

This type of aragonite has come from Spain as clusters in reddish clay rich in gypsum and celestine. They are also common in several other localities and are easily collected; however, they are not particularly valuable because of their abundance and unimpressive appearance. An exception comes from a large sedimentary deposit at Corocoro, La Paz County, Bolivia, where small clusters of what were originally hexagonal sixling aragonite have pseudomorphed and have been replaced by native copper. These are most unusual and highly prized by copper collectors.
Sicily As A Source
One of the great sources of aragonite of the past several centuries that is still viable today is the Island of Sicily. Sulfur from this location was used in Roman times and perhaps before that. There are many mines in Sicily closed now that have yielded countless specimens of sulfur and related species. The most prolific mines include Cozzodisi, Racalmuto, and Cianaciana and Agrigento, all in the region of Sicily. In the 1970s, this writer enjoyed trading with a fellow who lived near the Sicilian mines to obtain some very fine fluorescent specimens and showy sulfur and aragonite specimens.
The aragonite from this part of the world is also associated with bright yellow sulfur crystals, fine celestine crystals, water-clear selenite gypsum crystals, and small, sharp dogtooth calcite crystals. It is not unusual to find sulfur crystals enclosed by selenite crystals. Small, sharp calcite crystals are commonplace on aragonite, and celestine crystals tend to be found in fan-like clusters with all other species. In addition, a relatively soft, sometimes crumbly deposit of clayey material of calcium carbonate composition is often the matrix for these minerals, all of which fluoresce.
The aragonite found in Sicily’s mines also tends to occur in flat, disc-like, pseudohexagonal crystals attached to each other in a platy arrangement with each crystal measuring up to 2 inches across. Plus, the aragonite is often topped by tiny, sharp calcites. The aragonite prism faces of each crystal are very short and hardly visible. These hexagonal discs are seldom more than an inch high. The other common type is lovely, nearly transparent, individual pseudohexagonal crystals that measure up to an inch across and more than an inch long. They develop in nice sub-parallel clusters of high luster and sharp terminations.
Fluorescence Focus
One of the exciting features of all five of these Sicilian minerals is their fluorescence. The exception is the sulfur itself, which does not respond to ultraviolet excitation. The aragonite glows a greenish-white and phosphoresces with a nice white glow. The calcite often responds as a delicate pink and the celestine a subtle white.
While aragonite is found worldwide in marvelous crystals of several types, perhaps its most important function is somewhat hidden from view but is present in the vast majority of sea life that depend on carbonate minerals for structure and appearance. Aragonite is a critical component of sea shells that sea creatures make and need. It is also essential in the making of natural pearls. It is part of the lovely mother-of-pearl lining of seashells, which is a popular lapidary material found in a variety of colors due to natural impurities.
Ancient History
The dawn of aragonite’s importance dates back several hundred million years to the time when sea creatures were beginning to create shells. As soon as soft sea life began building hard shells, both aragonite and calcite played an important role.
Sea creatures use any one of three materials to construct their protective shells. For example, silica is important to tiny creatures like diatoms. Some larger sea creatures also use silica, but calcium carbonate is by far the most important ingredient in protective and useful sea life shells.
It should be noted that these two minerals can convert one to the other, but calcite is the more stable.
Ties to Seashells

During the evolution of seashells over millions of years, each type of creature developed its own system for creating a shell or a shell lining. Most creatures used both calcite and aragonite in constructing their shells, depending on environmental conditions. It is interesting to note that in some cases the shells of creatures dating back more than 75 million years that contained aragonite have often polymorphed into calcite.
For fossil collectors and jewelry makers, the most outstanding examples of aragonite in ancient creatures can be seen on the surfaces of some ammonites and in lapidary material derived from ammonites, which is known commercially as ammolite.
Canadian Deposits
A large deposit in Canada near Lethbridge has been producing spectacular and very unusual ammonites often visible during exhibits. The best of these are largely complete coiled shells of the ancestors to the squid and octopus. The outer surface of these unusual ammonites is often celebrated for its beauty, which is due in part to the fossil surface being covered with a thin layer of aragonite. The layer acts as a diffraction grating that splits light into its component colors when it strikes the surface of an ammonite. The most common visible colors are red, green, and orange. The colors blue and violet may also be seen but not as often. All are quite spectacular. Properly cleaned and lit, these amazing ammonite fossils are a spectacular museum-quality example of the action of tiny aragonite platelets in splitting light.
Ammolite is fragments of ammonites that were found in the same formation where complete ammonites were found near Lethbridge. The small fragments of material that have flaked off broken ammonites are carefully backed in one of several ways and used in jewelry as a very colorful stone. Ammolite produces the same spectacular array of colors as seen in ammonites. Additional gems dependent on aragonite are pearl and mother of pearl found in both salt and freshwater bivalves!
Pearl Growth Process
The growth mechanism of a pearl is fascinating. Starting with an irritant like a bit of sand that gets in the bivalve, the creature begins secreting calcium carbonate in the form of calcite along with organic material. Alternating layers of aragonite, nacre and organic material comprise the mass of the pearl. But that is only the beginning. The more valuable pearls have to have what is called color, iridescence, and orientation. The organic material adds to the color of a pearl. Nacre is responsible for the iridescence and orientation or reflection of light. Nacre is regularly secreted in parallel layers of aragonite platelets deposited as single crystals in a regular structure. When nacre covers the inner surface of a shell, we call it mother of pearl. Without aragonite, the mother of pearl would lack the inner glow so many find attractive. The same is true of fine pearls whose iridescence is due to aragonite nacre.
While aragonite can excite crystal collectors, this orthorhombic calcium carbonate plays a much more critical role in creating valuable lapidary material and lovely organic gems. Either way, it is an important collectible material.
This story previously appeared in Rock & Gem magazine. Click here to subscribe. Story by Bob Jones.