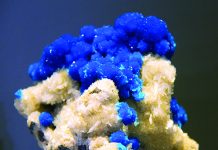
The fluorite mineral is a favorite among collectors. Fluorite crystals are often large, well-developed with a rainbow of colors, relative abundance and affordability. But fluorite has something else going for it. Fluorite has a history as fascinating as its mineralogy.
A Smelting Flux

Fluorite’s association with silver and lead ores first brought it to the attention of Roman miners. They found that it improved the smelting process by reducing melting temperatures while combining with impurities into an easily removable slag.
German smelter workers of the 1500s also used fluorite, then known as Flusse or Flusspat, as a smelting flux. In his classic “De Re Metallica,” German scholar Agricola (Georg Bauer) detailed the use of Flusspat, which he called “lapides igni liquiscentres.” This translates to “stones that become liquid in fire.” Agricola also referred to the mineral as Fluere from the Latin fluor meaning “to flow.” This is the origin of our modern word, fluorite.
Etching Glass
Glass was thought to be impervious to all liquids until 1670 when a German glassworker mixed ground Flusspat with sulfuric acid in a glass container. The glassworker was astounded when the glass dissolved. The glass-dissolving agent was a secondary acid that became known as Flusspatäure, literally “acid of Flusspat.”
The ability of Flusspatäure to etch glass brought new creativity to the art of glassmaking. More importantly, it indicated to chemists that both Flusspat and Flusspatäure contained an active, unknown element. The identification and isolation of this element became the goal of leading scientists for the next century.
The “Fluorine Martyrs”

Wikimedia Commons.
Although not yet isolated, this mysterious element was named in 1813. French physicist, André-Marie Ampère, acknowledged the element’s hazardous nature. He proposed the name “phtor,” which is the Greek word for “destructive.” English chemist Sir Humphry Davy adhered to tradition and coined the word “fluorine” from the old Latin fluor. In 1826, scientists adopted the words “fluoride” for fluorine-containing compounds and “hydrofluoric acid” for Flusspatäure.
Unfortunately, chemists seriously underestimated the dangers of fluorine. More than a dozen researchers lost their health or their lives by inhaling or contacting elemental fluorine or fluorine-based vapors. They became known as “fluorine martyrs.”
In 1886, French chemist, Frederick Henri Moissan, isolated pure fluorine by electrolytically reducing a mix of hydrofluoric acid and potassium fluoride. He then demonstrated that the yellowish gas was the most chemically active of all elements. His work earned the 1906 Nobel Prize for chemistry. But Moissan paid dearly for the honor, dying a year later at age 55, the last of the fluorine martyrs.
From the A-Bomb to the Present
During World War II, large quantities of fluorite from the Illinois-Kentucky Fluorite District were shipped with false bills of lading via circuitous routes to nonexistent destinations to confuse any possible Axis spies. The fluorite’s true destination was a top-secret facility in Tennessee, where it was converted to elemental fluorine. It was then converted to uranium hexafluoride gas for the separation of the fissionable uranium-235 isotope needed to make the atomic bombs that ended World War II.
 Fluorite (calcium fluoride, CaF2) crystallizes in the isometric system and has a Mohs hardness of 4.0, a specific gravity of 3.18, and perfect, four-directional cleavage. It often occurs in hydrothermal veins and is a common gangue component of the sulfide ores of lead, zinc and silver.
Fluorite (calcium fluoride, CaF2) crystallizes in the isometric system and has a Mohs hardness of 4.0, a specific gravity of 3.18, and perfect, four-directional cleavage. It often occurs in hydrothermal veins and is a common gangue component of the sulfide ores of lead, zinc and silver.
Today, seven million metric tons of fluorspar (the commercial term for impure fluorite) are mined worldwide each year. Half is converted to hydrofluoric acid, a feedstock for numerous industrial chemical processes. Most of the remainder is used to smelt steel and aluminum. China now produces half the world’s fluorspar; Mexico and South Africa are also important sources.
Because of its strongly bound fluorine and calcium ions, the fluorite in our mineral collections is quite safe and stable. But never heat fluorite or treat it with acid or any other chemical. As the fluorine martyrs so tragically learned, there is much more to fluorite than meets the eye.
This rock science column about fluorite minerals previously appeared in Rock & Gem magazine. Click here to subscribe! Story by Steve Voynick.