Iron ore minerals are the naturally occurring sources of iron, from magnetic magnetite to non-magnetic hematite and siderite. These minerals not only fueled the Iron Age but also shaped our modern world, from steelmaking to scientific discovery.
Iron Ore Minerals in History and Industry
Iron ore minerals have played a major role in human history. After the Bronze Age came the Iron Age, when the smelting of iron ore revolutionized tools, weapons, and building materials. Today, vast iron ore deposits fuel a global steel industry that builds our cities, ships, and infrastructure. Mining operations in regions like Australia’s Pilbara and Minnesota’s Mesabi Range still extract millions of tons of ore each year to meet demand.
Iron also plays an important role in our health. We are red-blooded thanks to the atoms of iron in every red blood cell. Iron grabs oxygen, carries it where needed, and releases it. Without it, we would be anemic and die.

Ekaterina/Adobe Stock
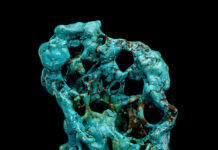
Some iron ore minerals, like magnetite or lodestone, are strongly attracted to a magnet. Others are not. Minerals such as siderite and hematite contain iron, but the iron is bound in a different mineral compound, making them non-magnetic despite their importance as iron ores.
Understanding Magnets and Magnetic Force
We know that for a magnet to work, things must line up internally. The “things” are electrons, which have to spin in the same direction, creating a north-south influence. This is why nails, or needles, become magnetized from electricity, which flows in one direction.
Stroking the needle in one direction with a magnet will magnetize it. But once formed, are magnets permanent? Over time, a magnet’s strength may slowly weaken. Hitting or dropping it, will disrupt the electron spin and disrupt the alignment of electrons and magnetism.
The same thing happens with magnetite. Its electrons all spin in the same direction. Aligning in the same direction dictates the strength of the magnetism. The electrons of an element, like iron, also influence the chemistry of minerals.
How Iron Ore Minerals Form

All elements are composed of protons and electrons. Forming a mineral requires an element acting as a metal, called a cation, which gives up its electrons to form a mineral. Iron does this and is positively charged as a result.
To form a mineral compound, a non-metal, like oxygen, takes in electrons and becomes negatively charged. This negatively charged non-metal oxygen joins the positively charged iron to form a mineral compound.
Iron and oxygen can join in a variety of ways to form compounds, so we get different iron minerals, magnetic magnetite and non-magnetic hematite, siderite ad infinitum and even rust.

Annda/Adobe Stock
Magnetite: A Key Iron Ore Mineral
Most mineral collectors are familiar with magnetite, a natural magnet and one of the world’s primary iron ores. Magnetite is mined extensively for steel production, with major deposits found in Australia, Brazil, and North America. It tends to be black in color and lustrous, and its crystals form in the isometric system, often as octahedrons. It more often develops in octahedrons and less often in 12-sided dodecahedrons. Magnetite occurs in many rock types, from igneous to metamorphic, and can appear as grains, small crystals, or large, well-formed specimens.
Fine examples of octahedral magnetite have been dug by rockhounds on Twin Peaks, Millard County, Utah. The octahedrons are usually under an inch but form sharp clusters. Historically, magnetite played an important role in navigation when naturally magnetized specimens, called lodestone, were used to indicate direction. named “lode,” which means “journey.”
Lodestone: Naturally Magnetized Iron Ore
During the second century B.C., early people were naturally curious about lodestone because of its magnetic property. In China, they found that if they floated a lodestone needle on water, it would indicate direction. A liquid “compass” wasn’t used on a ship, but if the needle was suspended in air, it would indicate direction.

A compass works because the earth itself is surrounded by a life-saving magnetic field. What’s very interesting, and maybe scary, is that we know the Earth’s magnetic field reverses. We just don’t know why or know the consequences.
Be that as it may, it’s a good thing the Earth has a magnetic field because it protects us from most of the lethal radiation constantly discharging from the sun. Some of that radiation, like ultraviolet rays, does penetrate. But the Earth’s magnetic field makes life as we know it possible.
While lodestone itself is relatively rare, its parent mineral, magnetite, is an abundant and valuable iron ore extracted on a massive scale today. This ore feeds the steel industry, which shapes modern infrastructure and technology.
Iron Ore Minerals in the Earth’s Crust
Since we depend on the Earth’s magnetic field, we need to know what produces this life shield. To understand, we have to look at the Earth’s core. We can’t peer that far into the earth, so we must use other means. Earthquakes are one way to “look” into the Earth.
An earthquake causes the entire Earth to vibrate. The rock structures of the earth can slow, speed up, or deflect those vibrations, which tell us something about the rock layers within the earth.
From this, we are convinced the Earth’s innermost core is a solid nickel-iron suspended in an outer semi-liquid nickel-iron-rock core. As the Earth rotates, the inner core rotates more slowly, creating that all-important magnetic field.
If we are right about the Earth’s core, where did the iron come from? The general theory is that the Earth was formed by accretion, the repeated crashing together of individual rocky masses, comets, and meteors as gravity pulled them in. That material was leftover from ancient exploding stars.
Note that iron, atomic number 26, is the heaviest element that a star like ours can create by atomic fusion. Gravity pulled all this space debris together to form the Earth 4.6 billion years ago, and we are still growing.
During the Earth’s early history, abundant iron in the crust combined with oxygen produced by early photosynthetic life formed vast iron oxide layers. These ancient formations are still mined today, supplying the raw materials for steelmaking worldwide.
Formation of Major Iron Deposits
So why are the heavier elements like gold not deep in the earth? We find them in the crust. Luckily, crustal movement caused by great internal heat causes super-hydrothermal solutions to bring the heavier elements up into the crust, and we mine them!
During the Earth’s early millennium, the crust was rich in meteorite iron, while the Earth’s early atmosphere was methane, ammonia, and carbon dioxide. Then an organic life form called stromatolites developed photosynthesis, which takes in carbon dioxide and produces oxygen, changing the atmosphere. Oxygen, combined with the available iron in the crust, forms massive amounts of iron compounds like rust, hematite, siderite, magnetite and other iron minerals.
We now find these huge iron oxide deposits and mine them in Australia, Alabama, Minnesota and elsewhere. The huge Mesabi iron range, among others, are what made America a huge steel producer.
We have to be grateful to magnets and the Earth with its magnetic field. Its slowly rotating nickel-iron core has made life possible on earth and protected it so we can enjoy the benefits of our red blood cells as we collect iron minerals magnetite, hematite, and siderite, all the while dealing with annoying hydrous iron oxide: Rust!
This story about iron ore minerals appeared in the August 2021 issue of Rock & Gem magazine. Click here to subscribe! Story by Bob Jones.