
Finding native silver is not as easy as finding native gold when it comes to naturally occurring precious metal specimens on Earth. The general belief that gold is hard to find and rare is certainly true. Another common opinion is that gold is rarer than silver, which it is. But, that same thought does not apply to crystallized gold specimens that naturally occur and naturally occurring silver specimens. In this comparison, native silver is rarer.
 Gold is certainly many times more costly per ounce than silver. Most of us can’t afford to eat with gold utensils, but every home has silverware as utensils and decorative objects. We wear all sorts of silver jewelry, from belt buckles to necklaces decorated with gems.
Gold is certainly many times more costly per ounce than silver. Most of us can’t afford to eat with gold utensils, but every home has silverware as utensils and decorative objects. We wear all sorts of silver jewelry, from belt buckles to necklaces decorated with gems.
Visiting the Silver Vault
On one of my visits to the Natural History Museum in London, Museum Curator, Pete Embrey, suggested I visit the Silver Vault, about which I had never heard. When my wife, Carol and I visited the vault, we were astounded. We were allowed to visit the lower level of a building near Harrods.

After passing a guard and security cameras, we walked into a long hall lined with vaulted rooms like a bank. Each room was filled with sterling silver objects such as candelabras, huge bowls, trays, tea serving sets and all sorts of animal sculptures, including large swans and ducks done in sterling silver. These items had been the property of the wealthy of England in the great days of England’s Empire. It was an amazing sight to see so much sterling silver. If you make it to London, make a point to go see all that wealth in sterling silver!

Native Silver Rarer Than Native Gold?
After I painted that picture, would you believe that native silver specimens are rarer than native gold specimens? Actually, we have found gold in more or less pure form ever since humans picked up the first yellow nugget. How many times have you heard of someone picking up a shiny silver nugget? Even when native silver is found, it may not look much like its natural shiny white color, thanks to its tarnish. It is most likely black.
You simply don’t find native silver scattered about, causing a silver rush to happen and prompting people from all over the world to flock to the destination to mine silver. That has happened countless times when native gold has been found, as in Alaska, California, Australia, South Africa, and who knows where else. There have been rushes to stake claims on silver deposits like the Comstock Lode of Nevada, but even then it started as a gold discovery.
The Comstock Lode
The Comstock Lode was the first and biggest silver discovery in America. Native silver was found, but the ore was argentiferous ore and silver compounds, not native silver.
The deposit resulted from volcanic activity that injected rich silver ores between diorite and andesite formations on Mt. Davidson. The veins were six rich shoots of unstable ore and constituted the bulk of the Comstock Lode. Mining in the area was hazardous because the host rocks were treacherous and frequent collapses created problems and deaths. Luckily, a German engineer, Phillip Deidesheimer, developed the square-set timbering method that is still in use today. The invention is a framework of interlocking timber cubes that provided roof support and a working platform for the miners.

Native Silver at the Comstock Lode
Unlike gold, which is almost never combined chemically with anything, silver almost always is in compounds. It has to be torn away from the other elements it joins. These silver minerals are familiar to many; proustite, pyrargyrite, acanthite, polybasite and others. Native silver was found in the Comstock Lode, but it was not the main ore. One old report described a pocket with silver wires in it with red proustite crystals hanging from the wires. That must have been some sight!
When this deposit was first worked, it was for gold, but the miners complained about the heavy black sand that messed up their gold recovery. Of course, this proved to be silver ore, and that’s what started the big rush.
Extracting silver from Comstock’s ores in the 1800s was not easy. Miners used a process developed in Germany’s silver mines, but a lot of silver was lost. Finally, a new smelting method called the Washoe Process, now obsolete, was used and much more productive. Almost all silver deposits found do not produce a lot of native silver specimens. The exceptions are places like Kongsberg, Norway, some of the old German silver mines, as well as the Michigan copper/silver mines. Native silver is rarely discovered, even today.

Kongsberg, Norway & Native Silver
Of all the deposits that have produced native silver specimens in quantity, Kongsberg, Norway, is the leader. It has been the largest deposit of native silver ever worked with some 200 mines operating at one time.
The neat thing about this deposit is it was not discovered by a person but an ox! In 1632, two youngsters were tending their herd, and an ox decided to scrape the coating off his horns and rubbed them against a rock, exposing a shiny metal. The children brought some of the metal home to their dad, who realized what it was. He collected some of the silver and sold it, only to be later arrested for stealing the king’s property!
Dynamic Deposits
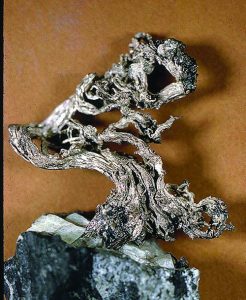
Kongsberg Mines
The Kongsberg mines were a rich source of native silver and a variety of silver compounds. Much of the silver was in calcite. Large veins were worked as far down as about 3,000 feet. The beauty of this deposit was that the metal could be melted directly. For collectors, the appeal is the great variety of silver specimens — from superb, perfect cubes through distorted crystals to long curling wires as long as an adult’s arm and as thick as your thigh. Some examples are on display in the Norway Mining Museum in Kongsberg.
Michigan Copper Mines
The Michigan copper mines also produced wonderful native silver specimens closely associated with native copper crystals since they crystallized together. Specimens of silver from this region are always well-crystallized, often as slightly rounded dodecahedrons. Most specimens are thick interlocking tangles of wires, distorted crystals, and crystallized masses in various shapes. The mines in Michigan are closed, as are those in Kongsberg, but Michigan locals using metal detectors and venturing underground are finding some silver specimens.

Silver in Arizona
Years ago, two metal detector prospectors came across large lumps of native silver in Arizona, an exceptionally rare find. The largest silver mass weighed 417 pounds, which was the largest silver nugget found anywhere in generations. Compare that with the 2018 find of gold in Australia, where many huge masses of quartz and native gold were encountered during nickel mining – the largest quartz piece had several thousand ounces of nearly pure gold in it, and all the quartz specimens were shot through with gold from the same “pocket” or vein totaling millions in native gold. One specimen from this find had its quartz removed to expose more gold, and the specimen was shown in the 2020 Tucson Gem & Mineral Show® and is now in the Perot Museum in Dallas.
Gold & Silver-Bearing Minerals
The majority of silver deposits produce silver-bearing minerals such as chlorargyrite, acanthite, poybasite, prostite, pyrargyrite, silver-rich galena, tetrahedrite, bournonite, sphalerite and other species that accommodate silver.
From California alone, millions of dollars in native gold from dust to big crystallized specimens have poured forth since 1848, and almost all of it is native gold. Only in deposits like the mines at Cripple Creek, Colorado, have gold come from the ground, combined with calaverite and sylvanite elements. It will also combine loosely with some of the halogen elements and cyanide, hence the cyanide process for recovering gold particles. Once attached to the cyanide, gold is quickly released when zinc is introduced.
Noble Elements
Gold and silver, along with platinum, are considered noble elements. Gold is noble because it remains aloof from almost all other elements. It forms alloys with mercury and silver, developing electrum, but rarely combines chemically with other elements.
Gold will not tarnish, which explains why King Tut’s funeral mask was still brilliant after thousands of years underground. You can even say that about any native gold mined. It is still just bright yellow native gold!
You can’t say that about native silver. Even when we find native silver during mining, it’s lovely bright silvery color is masked because silver tarnishes, combined with sulfur or other elements, given time. So why is silver a noble element? It is not because it remains aloof from other elements. It is simply rare enough to be considered noble.

Why Are Native Silver Specimens So Rare?
When we consider these two precious metals’ chemistry, we find out why native silver specimens are rarer than native gold specimens. Remember, mineral compounds form when elements give up, share, or take in electrons to form a mineral. Each of these noble elements has one electron in its orbital shell. You would think these would be given up easily. In silver, it is, but gold does not give up or take in electrons except in rare telluride cases we mentioned.
The chemical differences between gold and silver are apparent in the electronics industry. In circuits that must remain unaffected by tarnish or chemical change, gold is used. Silver is never used in the most sensitive modern circuits because it is chemically active.
Useful Silver
Silver stands out among the metals for other useful properties. Of all the metals, silver is the best electrical conductor. The conductivity of a metal depends on a metal atom’s electrons’ ability to move to create an electrical current. Copper, gold, and silver all have just one unpaired electron in their final orbital shells and, as such, are excellent conductors. The most considerable tonnages of pure silver pouring from smelters worldwide have to be separated from silver compounds, not native silver. Smelting of gold is mainly a function of separating gold not chemically combined with other elements but locked in the rock where it formed.
Aside from Kongsberg, Michigan, and a few other places, what is remarkable are native silver specimens recovered from the old German silver mines over a thousand years ago that are still with us. They are present today because royalty and nobility treasured the specimens, and they ended up in museums and private hands. These specimens show up rarely today, so if you have enough gold, you can buy a nice piece of native silver.
This story about native silver appeared in a previous issue of Rock & Gem magazine. Story by Bob Jones. Click here to subscribe.















