Story and photos by Lauritz A. Jensen, with photos also by Hans Gamma
Few hardcore lapidaries and mineral hobbyists would ever consider passing up a semiprecious gemstone that is flushed in scarlet, vermilion, or magenta. Or would they?
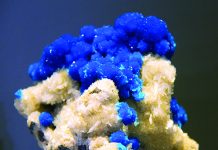
Even though color is everything for many mineral collectors and jewelry designers, many often worry about dangerous substances found in rocks—especially those drenched in crimson. Shades of reds and purples, which are characteristic of cinnabar, are without question captivating and eye catching—absolute crowd pleasers. The obvious problem here is that a silicate that profiles this colorful ore is actually a rock filled with poisonous mercury inclusions, specifically in the form of mercury sulfide (HgS).
Understanding Mercury’s Presence
To be sure, mercury disturbs many rock enthusiasts, and causes many to be squeamish about working with it. Even the most pleoperfect, chromatic gemstone might not be reason sufficient to grind and polish it into a cabochon.

To complicate matters, rockhounds often mistakenly believe that all material from abandoned mercury mines and dumps consists of nasty minerals that are detrimental to one’s health. But, sometimes apprehensions are exaggerated or misplaced altogether.
Indeed, many jaspers and agates from old repositories bear only a superficial resemblance to cinnabar and actually harbor no substantial quantity of mercury. Instead, iron, manganese, and chromium serve as coloring agents. Consequently, these cinnabar companions are resplendently elegant rocks, which are more than suitable for even the most discriminating lapidary to shape and polish.
In this article we will identify and describe the mercury-bearing and associated or companion rocks from the Sunflower Mine region of Arizona.
Sunflower Mine’s Offerings
Like many sections in the Mazatzal Range of the Tonto National Forest, there is visible evidence of ancient volcanic activity and marked sedimentary processes, and as is frequently the case, fascinating rocks and minerals abound in this type of environment.
To put it succinctly, the Sunflower Mining District pullulates with colorful stones, and although there are many wonderful choices for the lapidary arts, red cinnabar is undeniably the main draw—conceivably a bi-product of recent geologically thermal spring activity. A dark gray variety of mercury sulfide, metacinnabar, also is present.
Mercury is traditionally classified into three forms: elemental or metallic, inorganic, and organic compounds. Historically, elemental mercury was referred to as quicksilver in industry, dentistry, and mining communities. Because of its amalgam property, it was an invaluable commodity for the extraction of gold as it allowed for cost-effective recovery—especially fine granules mixed in sand.
Mercury’s Differences
Moreover, it served as a critical component in thermometers and gravity switches. Of course, it is still used in dentistry but to a much lesser degree than a few years ago, primarily because of the popularity of ceramic crowns and composite resin fillings. Many of us, especially baby boomers, were encouraged by our science teachers to handle the liquid silvery metal and discover firsthand some of its dynamic physical properties. The supremely dense liquid metal was certainly fascinating to look at as it permitted a silver dollar or a lead weight to float on its surface—an impressive demonstration of density that surely caught the attention of even the most bored student. Pouring it out on the tabletop and watching the droplet spheres instantly coalesce into larger blobs was also a fun activity, but it was soon recognized that this metal was one of the most toxic substances on earth.
Fortunately for us, elemental mercury is poorly absorbed through the skin, and

its bioavailability is greatly diminished in the gastrointestinal tract. Mercury in general has a reduced solubility in water, but inhaling the vapors, nonetheless, has an effect on the nervous system.
As stated, the mineral cinnabar owes its toxicity and vermilion color to the inorganic compound mercury sulfide (HgS). This ore serves as the main source for quicksilver, which is routinely sweated out of the rock by means of a furnace-retort system. Remarkably, rich ore has been known to spontaneously emit the liquid metal with droplets appearing on its surface.
Respecting Cinnabar
Cinnabar is believed to be commensurably safer than the liquid metal and is considered to a limited extent okay to handle. Still it must be respected. The perils of freely breathing air charged with HgS (cinnabar dust)—perhaps by unprotected digging in a vacated mine or an abandoned retort site—is less than rational. The gradual accumulation of inorganic mercury clearly leads to renal and neurotoxicity. Of note, excessive salivation or ptyalism is a sign that the person suffers from mercury poisoning, but this seldom thwarted the most disadvantaged folks from working mines in times past. In truth, this was a fairly common manifestation among Mexican and Chinese miners during the Sonoma quicksilver rush of 1873–1875 in California (Quicksilver Mining in Sonoma County, p. 45, by Joe Pelanconi, The History Press, 2014).
Many organomercury compounds are actually critical ingredients of insecticides and fungicides. Others are helpful as topical antiseptics or preservatives for intravenous drugs and certain vaccines. For instance, the use of Thimerosal (ethylmercury) in medicine, although controversial, has value in that it thwarts microbial growth in the multi-dose vials of trivalent and quadrivalent influenza vaccines. Syringe needles typically enter these vials multiple times, potentially contaminating the vaccine product with bacteria, but ethylmercury greatly reduces this contamination—though there is no point to use it in single-dose vaccines.
Regarding the environment, organic mercury compounds (e.g., methylmercury) pollution is real. Nonstop discharge of industrial waste such as what took place in Minamata Bay in Japan in the 1930s through the 1960s, for example, has been linked to severe mercury poisoning in people including neurological manifestations and hundreds of associated deaths. To be factual, many natural sources of mercury also exist (e.g., volcanic eruptions, forest fires, and the volatilization of seawater). Anaerobic microorganisms in the environment have the ability to methylate or convert elemental and inorganic mercury into an organic form that will enter the food chain and accumulate within the body.
Mercury Mines
People may become exposed by consuming contaminated fish, including freshwater fish. Of note, the Arizona Department of Environmental Quality has issued fish consumption advisories due to mercury contamination for nearly 20 lakes and reservoirs (ADEQ Fact Sheet, Publication Number: FS 15-09, Revised 08-17). Evidently, there is a lot of mercury in the soil throughout Arizona—either due to industrial pollution or perhaps just because it is naturally present in the earth.

For a time, mercury mines proliferated in several areas of Arizona. One of the most famous mines in Phoenix, the Rico Mine, is now paved over—mostly by AZ 51. The area was known as Dreamy Draw—undoubtedly a reference to the neurological side effects of breathing too much cinnabar dust.
Being confused or spacey was apparently an accepted norm for working in the mine. It seems that a substantial mercury belt also occurs in another area of the state, chiefly the Mazatzal Mountains. Here a lot of mining claims and processing sites are referenced in geological survey bulletins of the U.S. Department of the Interior.
Although we should not over stress about what occurs naturally in the earth, the upshot for lapidaries and mineral collectors is to take a no-nonsense approach when visiting deserted mining sites and while grinding the collected specimens. Without question, it is important to protect oneself by not breathing rock dust in any form. The dirt in the immediate vicinity of exhausted Arizona mines is likely contaminated with mercury sulfide, arsenic, and other toxic metals.
Sycamore Creek’s Legacy
In years past, one of the more prolific and easiest spots for collecting cinnabar in Arizona was the Sycamore Creek region in the Mazatzal Mountains of the Tonto National Forest, which is approximately 4,500 feet in elevation and immediately adjacent to a wilderness area. The old Sunflower Mercury Mine (AKA, National Mine), a lode deposit, was located here.
In 1911 E. H. Bowman, while prospecting for gold, recognized several rich deposits of mercury ore. He promptly filed six claims: Native, Packover, Titanic, Jasper, Go By, and Lone. Within a few years the Sunflower Cinnabar Mining Company purchased all his claims, and later acquired nine more from other miners in the district. A retort furnace was built in 1913.
The most productive period for mining occurred from 1913–1965, with quicksilver flasks (76 pounds per flask) in the thousands being produced from the ore. The Sunflower Mine was mostly an underground operation. Although meager in comparison, the mine also produced gold, silver, and copper. When the price of mercury dropped in value and health concerns grew in the 1980s, the operation completely shut down.
A fire in 2012, which was started by an incendiary shotgun blast, destroyed many of the wooden structures of the old processing and milling operations. The wooden structures that survived the fire were then removed by forest workers. A large rusted trammel, the most prominent relic of the quicksilver processing plant, and some scrap iron still remain; however, most of the other metal milling equipment have vanished entirely.
Limiting Access
As a protective measure, the actual mine entrance has been partially barricaded by the Tonto National Forest personnel. We did peek into one of the old adits, and while our flashlight lumens were not particularly bright, we could still see in the walls and ceiling the existence of a few mercury-specific quartz veins containing the speckled inclusions of cinnabar. At the request of the Forest Service, we did not seriously explore this or the other passageways. Apparently, the tunnels lack wooden supports and are in precarious states of decay—a good reason for putting aside our customary devil-may-care attitudes.
There are of course plenty of other reasons to deter the amateur explorer from inspecting abandon mines in Arizona, most notably because of the possibility of being overcome or perhaps outright dying from poisonous gases (e.g., carbon monoxide, methane, hydrogen sulfide, etc.).
In the Sunflower Mine, there was a substantial amount of water, which we were

guessing was greatly burdened with heavy metal that leached out of the surrounding walls. At the time of our visits, which occurred during late autumn, a gazillion cave flies stippled the entrance ways, probably merely seasonal denizens, but creepy just the same.
Moreover, a Tonto National Forest Officer informed us that there are many precarious and low-hanging rocks inside and possibly forgotten sticks of dynamite in these forsaken tunnels. Every so often a Western diamondback rattlesnake will find its way onto the mine property. This part of Arizona is famous for venomous serpents.
Mercury Mining Inventory
Other cinnabar mining activities in the immediate district include the Cornucopia, Oneida, and Pine Mountain Mines. In addition, the Ord Mine properties are located a few miles away. All are dangerous just the same. Explore around these mines, pick up a few rocks, take a lot of photographs, but resist the temptation to venture inside.
To get to the Sunflower or National Mine site, which is located about 35 miles from Fountain Hills, at Shea Boulevard take the SR 87 north to the Sunflower Mine/Mount Ord exit (.5 miles past mile marker 222).
Proceed on the paved road for 1.2 miles to a dirt road—more like a wide trail at this point—which is marked with a cattle guard. Turn right and proceed 1.2 mile to the next cattle guard.
Take a hard left on the trail which is now labeled Forest Service Road 25A on the map, but there is actually limited signage along the way. After three miles or so, the trail will take a sharp bend to the north paralleling the West Fork of Sycamore Creek and will continue for three or four miles.

Just driving along the trail will be a daunting activity. Heavy summer rains after the 2012 forest fire, destroyed thousands of acres of watershed and washed away the dirt surface leaving vast sections of the course embedded with only stones and jagged rocks. A four-wheel drive high-clearance truck or jeep is the minimal form of transportation for the trek, especially when passing through the streambed. In fact, a rock-climbing vehicle or an ATV is probably the most efficient way to pass over many of the rough spots. Technical off-road driving experience certainly has its benefits because in spots the course is rated a solid 9 out of 10.
Cinnabar’s Presence
Hiking the last two miles is a reasonable alternative. When you reach a fork in the trail—more or less a half mile past the bridge—take a sharp left and hike up the hill for another half mile to the old processing site. The actual mine is about 300 yards or so up a narrow canyon. Certainly, it is imprudent to explore this remote area unaccompanied, and keep in mind that this journey will probably not be a fun outing for young children or the faint of heart.
Before the 2012 Sunflower Fire, chunks of cinnabar were plentiful on the surface and amateur mineral collectors did not go home empty handed. Anne P. Fischer, Arizona On-Scene Coordinator, supervised the massive cleanup and remediation—a HAZMAT event. Analysis of the soil showed substantial amounts of mercury contamination, which prompted Tonto National Forest officials to construct an underground repository for containment and quarantine of the deadly dirt.
Presumably, a lot of cinnabar rocks were inadvertently buried in the process since they do not seem to be as visible as they once were. Nonetheless, cinnabar is still present in the canyon, but nowadays rockhounds will have to assiduously hunt for the stuff. Try in the vicinity of the mine and the old processing station, but again do not sneak around the portal barricades or crawl through the ventilation pipe to gain access to the tunnel. Cinnabar is not worth getting injured over.
If you decide to dig—and probably that is the best bet—protect yourself from the dust as it is likely to be contaminated with particles of mercury sulfide. A respirator mask might be worth considering. As an alternative, search the dry streambed of the West Fork of Sycamore Creek. In fact if you did not bring a shovel with you, the creek might be an excellent place to begin the hunt, as flash floods and heavy summer rains regularly uncover new material.
We have snagged many notable examples in the dry creek. A lot of specks or bits of cinnabar are sparsely distributed within quartz segments or veinlets, being sandwiched within chlorite-sericitic schist formations. This is when a good geology hammer comes in handy. Truth be told, microscopic particles of opal are mixed up with the quartz, a foremost signature for this rock. Cinnabar collected from this site is truly enthrallingly and exciting material—juggernauts of beauty—more than satisfactory for producing spectacular works of art.
Exploring Mines and Surrounding Areas
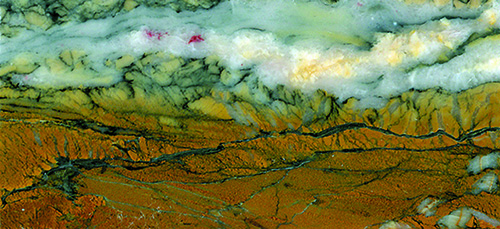
Companion silicates near the mine and surrounding hills are abundant and have definite lapidary value. Even if you are unable to bear the rigors of the difficult trail to the mine, you will find suitable lapidary material in the fields immediately adjacent to the roadway. The West Fork of the Sycamore Creek and washes are virtual argosies of bright red and reddish brown jasper and jasp-agate, and no one should go home with an empty bucket or knapsack. Indeed, the Forest Service reminds us that there is a voluntary 5-gallon bucket limit. When you think about it, that is a lot of rock, surely enough to keep the average hobbyist busy for months if not years.
Of special interest, although somewhat humorous, are the internet descriptions (also pics) of huge so-called cinnabar boulders in the streambed. Definitely, they are of prodigious proportions (hundreds of pound each), but to label them as cinnabar is a perfectly wrong identification. In reality, they are merely grandiose and textbook examples of jasper and jasp-agate. There is no visible cinnabar in these boulders, but they are astonishing just the same. Many are actually composed of broken segments of jasper that have been cemented together with a ferruginous pigmented limestone. This gives the rock a banded appearance.
We have come across dozens of super colossal archetypes of this red Arizona jasper in the washes and the main streambed, and it is fun to speculate just how many of these behemoths are still hiding beneath the surface of neighboring hills.
Besides these boulders, which incidentally are impossible to carry home, there are plenty of other rocks to collect. BIF (banded iron formation), for example, invariably features a prominent hematite carapace and occurs in schist-like layers no greater than two inches thick. Some of the reddish jasper is stratified, sometimes markedly so. Brecciated patterns also occur, possibly the result of a chemical transformation (i.e., disequilibrium).
The red jasper is regularly veined with milky white quartz stringers. As one would expect, the jasper and jasp-agate is composed respectively of 87% and 96% SiO2. Fe2O3, a ferric or iron(III) oxide, serves as the primary chromophore. Ferric iron and manganese gives the quartz, which is common in this jasp-agate, a cloudy look. A tight mossy-like pattern is probably the result of a secondary transformation. Tiny flecks of shiny hematite further adorn many specimens.
More Than Cinnabar
Also deposited in the area are pastel-colored pink quartz, bright maroon jasper, and brownish-orange jasper. The brownish-orange jasper is the hardest of all these silicates, and it characteristically takes a terrific polish—drop-dead perfection—that in our opinion rivals the shine of porcelain jaspers.
Furthermore, it features broken linear striations and curious globular reddish inclusions (fish eye bodies). Small agate druzies commonly show up in these slabs. The maroon jasper, a truly busy rock, may be layered with brighter reds which makes it especially captivating and prepossessing for jewelry and an assortment of other lapidary projects.
Red jasper deposits pop up in many regions of Maricopa and Yavapai Counties, and many novice lapidaries from Arizona have undoubtedly cabbed a piece or two, only to be disappointed—possibly because they were so dirty to work with and trend toward the dark hematite side of the color palette.
Although sometimes no cleaner, the reddish jasper from the Sycamore Creek area presents with immensely brighter reds. On the other hand, the jasp-agates are extremely clean to work up—arguably the exact type of rock that we live for as lapidaries. To achieve the high-grade polish that will be applauded by your buddies, we recommend using Tripoli pre-polish powder, followed with a premium grade of cerium oxide. This sequence is actually suitable for all silicate varieties found in the region. Interestingly, diamond powder has no particular advantage and actually slows down the polishing routine.
Occasionally, a super vug will emerge during cabbing, and it is terribly frustrating to encounter pitted specimens—always a letdown during the final phase of polishing. Regardless, do not overstress about the few blemishes that might occur from time to time because there is usually a remedy. For instance, hematite, which is the major chromophore here, is a congruent mineral, and the grinding residue or the dried powder when mixed with a good quality epoxy matches perfectly the true color of the stone.
Molecular Makeup
So, how safe are these companion rocks and what about their molecular makeup?
Much of this material does contain mercury, albeit in extremely tiny amounts. But, we recommend grinding wet and wearing respiratory mask and safety goggles just the same. If you are new to the hobby, it is sometimes difficult to determine if your material contains a toxic substance like an arsenical compound, asbestos-type minerals, carnotite, or beryllium. Point of fact, almost any metal will prove toxic especially if you breathe enough of it. Beyond debate, the unprotected grinding of jaspers or agates poses a substantial risk for hobbyists. Even an R95 painter’s respirator mask offers some protection against silica and other types of dust.
The scanning electron microscopy (SEM) is a precise instrument that tells us volumes about the attributes of rocks, including the crystalline structure and clues with regards to the elemental composition (via energy-dispersive x-ray spectroscopy or EDS). In our samples, EDS definitively identified significant amounts of mercury and other elements of interest in the some of the quartz but readings varied in magnitude, even when cinnabar inclusions were more than obvious.
Sometimes the milky white quartz zones were altogether devoid of mercury. SEM revealed that the quartz was often of a massive habit. Nevertheless, large euhedral crystals were predominant and noticeably well developed. Spherical structures of opal, probably opal-CT (cristobalite and tridymite) and zeolite crystals were prevalent. Technical assistance was provided by Dr. John C. Mitchell in the Midwestern University microscopy laboratory.
Imaging of the red jasper revealed the normal microcrystalline granular structure—predominantly euhedral crystals of approximately 3 µm in width. At this point, the opal-CT appeared to be in a state of transformation, presumably converting in the direction of jasper.
In our studies, we found that jasper was habitually preceded by opal. Pompoms and pellets (pompom conglomerates) showed up from time to time, but we were unable to determine whether these were remnants of opal-CT or merely a phase of jasper. Of specific interest were chains of fibrous or sprays of zeolite that filled the vugs. This type of zeolite (a large heterogeneous aluminosilicate group) most likely was a member of the natrolite family (i.e., mesolite, natrolite, or scolecite). At least one zeolite fibrous type, erionite is believed to be carcinogenic if inhaled.
Reviewing Evidence
Notwithstanding, there is currently inadequate evidence to suggest that human malignancies are attributable to any other zeolite. EDS readings in the red jasper samples did not detect any mercury, perhaps a limitation of the SEM microscope. But, the Agua Regia Digestion assay performed by ALS laboratories determined that the red jasper harbored trace amounts mercury (0.050 ppm). Tiny amounts (45 ppm) of arsenic were also present. See the table for a complete ALS analysis of the red jasper and jasp-agate.
Compared to the red jasper, considerably larger euhedral crystals characterized the jasp-agate. Pompoms also were observed.
Furthermore, ALS analysis revealed even smaller amounts of mercury (0.017 ppm) and arsenic (<5 ppm). Even though the SEM and ALS results are creditable measurements, our sample sizes were unquestionably limited. Further study of this lapidary material would be nice but costly and certainly impracticable from our perspective.
Perhaps one of the more rewarding projects for Sunflower cinnabar is a framed glitzy flat lapped representative—primo wall decor for the office or family room. Some show off landscape scenes. What is more, flat lapping carries few risks as the sanding and polishing is usually performed outside in the yard or garage away from most of family activities.
Tumbling the smaller chunks and then displaying them in a clear glass vase, either alone or with other polished pieces, is another idea. When tumbling, keep in mind that the mohs hardness of cinnabar is a mere 2.5—possibly less. If a rough specimen or a finished cabochon is desired, consider coating them with PermaLac® or another clear protective lacquer product in order to completely seal in all the mercury.
Collecting and Keeping Cinnabar
A floating glass locket is another idea. It should be noted, that as a result of the Minamata Convention and the associated international treaty, mercury mines around the world are now closing. New cinnabar slabs and rough will probably become virtually nonexistent in the marketplace within a few years. Eventually, the mercury surface rock from the Sunflower Mine region will completely disappear, and even though there is undoubtedly a lot of cinnabar buried deep in the surrounding hills, for the average rockhound to become a hard rock miner for the purpose of collecting a few pieces is unrealistic. Oh well!
The bulletin here is that companion silicates—nearly unlimited reserves of jaspers and jasp-agates—are measurably safer to work with than cinnabar and can be crafted into enchanting lapidary works of art every time.